Air dissolved in hydraulic fluid (not free air nor entrained air) is so elusive in the way it appears and disappears within the system that most hydraulic system designers are not familiar with dissolved air (or gases). Undoubtedly, however, they have experienced (to their dismay) the effects of entrained and/or free air in the system.
Dissolved Air Comes Out of Solution
Because air in solution in a hydraulic fluid has only a minor effect on hydraulic performance, why should the designers be concerned with it? The answer is that even if all the air bubbles and pockets were removed from a hydraulic system totally enclosed, after a short run-in period, air bubbles would begin to reappear. The source of these bubbles is the working fluid itself because all fluids (except fully deaerated ones) contain dissolved air that, under certain conditions, comes out of solution and plagues the system.
How hydraulic fluids “generate” air can be shown by the following test.
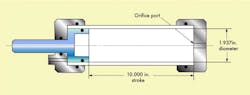
A conventional, double-acting actuator was connected to a 1-gpm, 3000-psi hydraulic system filled with MIL-H-5606 fluid, Figure 1. It was instrumented with a device, which measures the amount of entrained and free air in any air-oil mixture, Figure 2. Figure 3 is a device, used in tests described later in the article, to measure the amount of dissolved air in a fluid.
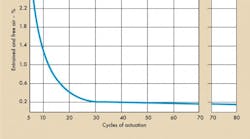
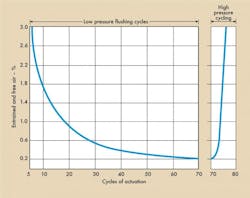
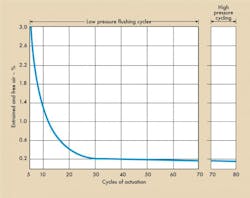
Starting with an empty actuator, flushing began at low pressure, about 300 psig. The cylinder was cycled with a 4-way valve with flow passing through an .0032-in diameter orifice at a cylinder port. The purpose of the orifice was to stimulate the area of a .0032-in diameter valve opening. After every six cycles, the test stand was shut down and an air measurement taken. Figure 4a illustrates the low pressure flushing cycles.
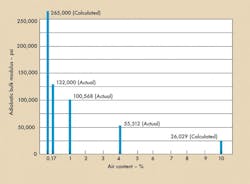
In addition, the cylinder was periodically pressurized to 1000,2000, and 3000 psig. The amount of fluid required to achieve these pressures was measured and recorded, Figure 5.a. The effective bulk modulus at 3000 psig was then computed for various air contents using the values obtained, Figure 5b.
It was startling to discover that, with a content of only 0.17% of compressible air, the effective bulk modulus was only half of the theoretical bulk modulus. The first inclination is to take solace from the fact that careful flushing had brought the compressible air content down to 0.17%.
After flushing, test stand pressure was increased to 1000 psig. After three cycles, the air was measured and found to be .08%. After six cycles, it was 2.6% and so on, Figure 4b. In short, dissolved air came out of solution and collected in the actuator. It is this form of air that negates normal fill and flush techniques.
Dissolved Air Increases Weight, Not Volume
It should be emphasized that neither the presence nor the absence of dissolved air affects the volume of the oil. And test data seems to indicate that there is no effect on bulk modulus, providing the air is in solution. These facts, at first, appear paradoxical. However, if one visualizes a container filled to the brim with marbles (which represent the oil molecules), it is possible to pour in fluid (representing air) around them, or remove the fluid with no change in volume. The weight of the container changes, not the volume.
When Entrained Air Appears
The appearance and disappearance of dissolved gases, in the form of entrained air, is an elusive phenomenon. Accelerated fluid through an orifice causes a local static pressure drop. If the pressure drops below atmospheric, dissolved gas is drawn out of solution and appears in the form of tiny bubbles (entrained air). If these bubbles do not conglomerate into larger bubbles, and if the velocity of fluid is low, most of the air bubbles are readsorbed down-stream where the static pressure is greater than atmospheric. This phenomenon agrees with Henry’s law.
An exception to this condition occurs when the fluid is accelerated above a velocity of 100 ft./sec. The air bubbles expand to larger size and are reluctant to go back into solution despite subsequent exposure to higher pressures. Erosion of materials has been known to take place in the vicinity of bubble growth. The reluctant of the bubbles to be readsorbed downstream appears strange at first until it is realized that the fluid immediately adjacent to the bubbles is undoubtedly saturated and cannot adsorb additional air.
When a stream containing bubbles is stopped suddenly, the bubbles will begin to migrate upwards and coalesce in the nearest high point. Repressurization may or may not drive the new, larger bubble back into solution.
Results of Air Removal
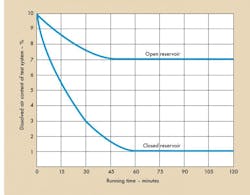
The hydraulic cylinder, described above, was tested while assembled in a system to evaluate the effects of air removal. A vacuum-type oil deaerator was operated for eight 15-minute cycles. A floating piston covered the reservoir fluid (“closed reservoir”) and the dissolved air content versus running time is shown in Figure 6. The actuator was then recycled at low pressures, as before, and air measurements were taken every six cycles, Figure 7.
System pressure was then raised, as before, to 1000 psig. Ten cycles were made to achieve a specific level of air content diminished (in Figure 7 compared to Figure 4a).
Conclusions
As a general conclusion, it is readily apparent that several areas of performance can be improved as a direct result of deaerating system fluid. Three other specific conclusions are drawn from these tests.
Effects of flushing. The effect of continuous, hard-over cycling upon air content agreed well with what could be expected intuitively. In fact, the final values achieved were far lower than what would be imagined for a cylinder with “built-in air pockets. Assiduous cycling can therefore be expected to effectively purge any given system of air.
Aeration due to dissolved air. The results of the high pressure cycling indicate that systems using air-saturated MIL-H-5606 fluid, or similar hydraulic fluids, at pressures of about 1000 psig or greater, will generate entrained air across orifices in the system.
Low pressure hard-over cycling of actuators can help remove the resultant air if the cylinder-to-valve lines are short.
Cylinders working unloaded and only in the mid-stroke range, with infrequent hard-over to hard-over signals, can expect increasing air contents with time.
Effects of Deaeration. Deaerating the system fluid prevented completely the generation of entrained air across the simulated valve orifice.
Previous tests, conducted with unsaturated fluid, indicated that accelerated purging also takes place because of the adsorption of small bubbles into the fluid. Further work is now underway to define qualitatively the reduction of pump nose and material erosion by use of unsaturated hydraulic fluid.
Caution With Bulk Modulus Figures
A word of caution is necessary. The values shown are not submitted as pragmatic numbers to be used with abandon. If anything, they point out that for any specific system, actual measurements should be made rather than rely on “text book” values.